-40%
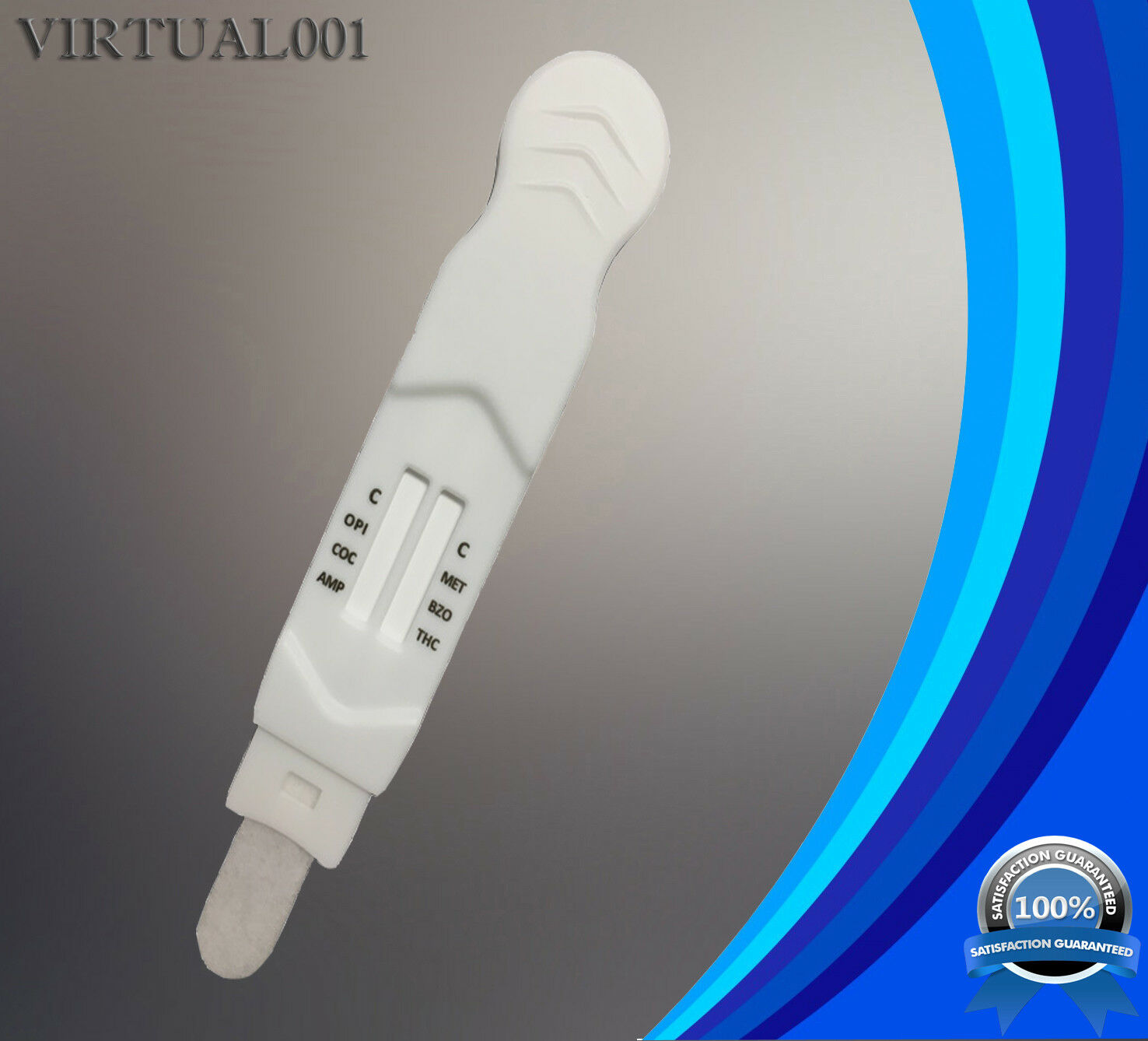
6 Panel Instant Oral Saliva Multi-Drug Test Kit
$ 4.21
- Description
- Size Guide
Description
Saliva Oral Fluid Direct Test for (6 Drugs)NEW DESIGN: Used like a thermometer!!!
The Most Affordable
One-Step
Oral Fluid Drug Test Device on the Market
Product Description:
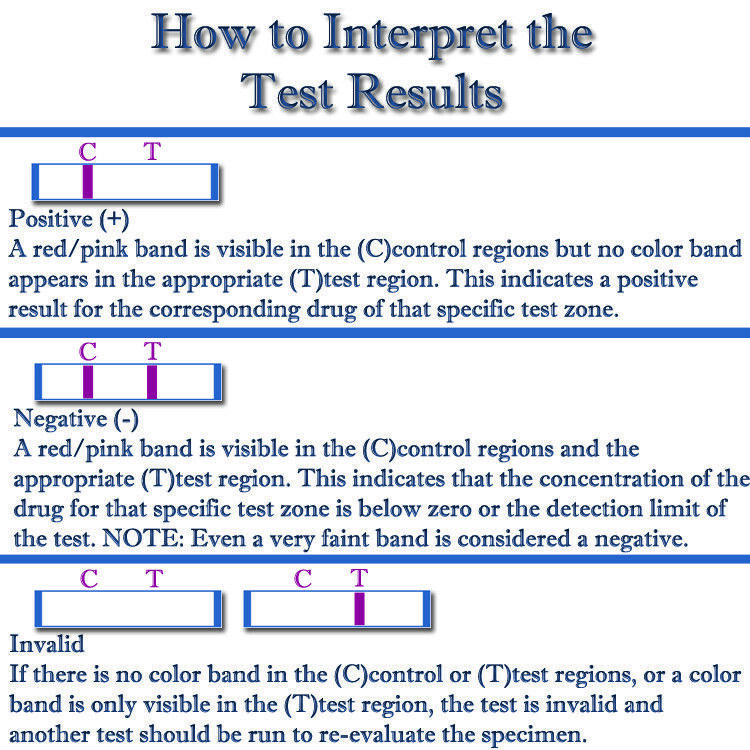
Appearance of red lines indicates sufficient collection of saliva
Screens for recent drug use
Non-invasive and eliminates gender observation issues
No bathroom facilities required
Solves the shy bladder issue
Difficult to tamper with or adulterate
Ability to supervise multiple test subjects at once
Simple one-step process with immediate results
Instructions:
Allow the test midstream to reach room temperature prior to testing
Confirm the person being tested has not ingested any food, drink, gum or tobacco for 15 minutes prior to collection
Remove the cap and insert the absorbent wick under the donors tongue for 3-5 minutes
A red line will appear next to the Control (C) region when the wick has absorbed sufficient saliva
Remove the device from the donors mouth
Place the cap back on and set the device on a flat level surface
Read results in 10 minutes. Results not valid if read after 1 hour
The list of drugs detectable with this test are as follows:
AMP- Amphetamine, 50 ng/ml - Adderall/Ritalin
BZO– Benzodiazepines, 30 ng/ml - Valium/Xanax/Paxam/Vicodin
COC– Cocaine, 20 ng/ml - Crack
mAMP– Methamphetamine, 50 ng/ml - Meth/Crystal/MDMA
OPI– Opiate, 40 ng/ml - Heroin/Morphine/Codeine
THC– Marijuana, 50 ng/ml - Cannabinoids/Weed/Pot
View more great items
On Jan-18-18 at 14:53:37 PST, seller added the following information: